18F-C2Am: a targeted imaging agent for detecting tumor cell death in vivo using positron emission tomography
2020.12.09.
Flaviu Bulat et al, EJNMMI Research, 2020
Summary
Introduction: Trialing novel cancer therapies in the clinic would benefit from imaging agents that can detect early evidence of treatment response. The timing, extent and distribution of cell death in tumors following treatment can give an indication of outcome. Here an 18F‑labeled derivative of a phosphatidylserine‑binding protein is described, the C2A domain of Synaptotagmin‑I (C2Am), for imaging tumor cell death in vivo using PET.
Methods: A one‑pot, two‑step automated synthesis of N‑(5‑[18F]fluoropentyl)maleimide has been developed, which was used to label the single cysteine residue in C2Am within 30 min at room temperature. Binding of 18F‑C2Am to apoptotic and necrotic tumor cells was assessed in vitro, and also in vivo, by dynamic PET and biodistribution measurements in mice bearing human tumor xenografts treated with a TRAILR2 agonist or with conventional chemotherapy. C2Am detection of tumor cell death was validated by correlation of probe binding with histological markers of cell death in tumor sections obtained immediately after imaging.
Results: 18F‑C2Am showed a favorable biodistribution profile, with predominantly renal clearance and minimal retention in spleen, liver, small intestine, bone and kidney, at 2 h following probe administration. 18F‑C2Am generated tumor‑to‑muscle (T/m) ratios of 6.1±2.1 and 10.7±2.4 within 2 h of probe administration in colorectal and breast tumor models, respectively, following treatment with the TRAILR2 agonist. The levels of cell death (CC3 positivity) following treatment were 12.9–58.8% and 11.3–79.7% in the breast and colorectal xenografts, respectively. Overall, a 20% increase in CC3 positivity generated a one unit increase in the post/pre‑treatment tumor contrast. Significant correlations were found between tracer uptake post‑treatment, at 2 h post‑probe administration, and histological markers of cell death.
Conclusion: The rapid clearance of 18F‑C2Am from the blood pool and low kidney retention allowed the spatial distribution of cell death in a tumor to be imaged during the course of therapy, providing a rapid assessment of tumor treatment response. 18F‑C2Am has the potential to be used in the clinic to assess early treatment response in tumors.
Results from nanoScan® PET/CT
Colo205 or MDA-MB-231 cells (5 or 10 million, respectively) were resuspended in 0.1 mL PBS or a 1:1 mixture of Matrigel and complete DMEM, respectively, and implanted subcutaneously in the upper back of 10–12 week-old female BALB/c Nu/Nu mice. Tumors were imaged when they reached ~ 1 cm3. PET/CT imaging was performed following injection of 18F-C2Am, which were performed in the same 2.5 h imaging session before and 24 h following treatment with MEDI3039 or chemotherapy (5FU or DOX, respectively). Helical CT data were acquired for anatomical reference and
attenuation correction (isotropic resolution of 0.2 mm). PET images, with a nominal isotropic resolution of 0.6 mm, were reconstructed using a 3D ordered subset expectation maximization (OSEM) method in one to three coincidence modes, eight iterations and six subsets. A 120-min dynamic PET acquisition was initiated 30 s prior to intravenous injection of 3.2±1.3 MBq 18F-C2Am, using a nanoScan PET/CT (Mediso) scanner. Scans were reconstructed into 23 time frames (4×15 s, from 0–1 min; 4×1 min, 1–5 min; 11×5 min, 5–60 min; 4×15 min, 60–120 min). Images were normalized and corrected for decay and attenuation and analyzed using VivoQuant software. Three-dimensional tissue regions of interest (ROI) were drawn manually, and, if possible, Otsu thresholding was applied to better delineate the ROIs. Standardized uptake values (SUV) were calculated as mean (SUV), median (SUV50), the 90th percentile (SUV90), peak (SUVpeak), peak_max (SUVpeakM) and maximum (SUVmax). SUV (g/mL) was defined as SUV=Cimg/ (IA/BW), where Cimg is the activity concentration (MBq/mL) in the ROI, IA is the injected activity (MBq), and BW is the body weight of the animal (in grams). The pixel with the maximum signal intensity was used to calculate SUV max. The fraction of injected activity per gram of tissue (IA/g, %), the tumor-to-muscle (T/m) and tumor-to-blood (T/b) ratios were also calculated, the latter two using the lower flank skeletal muscle and carotid
artery, respectively.
-
Biodistribution analysis showed that tumor activity post-treatment was at least ∼ 2-fold greater than in every other organ with the exception of kidney.
-
Renal retention (∼ 6% IA/g) was low and identical in both cohorts.
-
The uptake of 18F-C2Am was significantly higher (P< 0.05) in tumor, spleen and liver tissue following treatment, albeit the increase was smaller (~ 2.5 ×) in spleen and liver, compared with tumor (~ 5 ×) (Fig. 3).

Fig. 3 Biodistribution profile of 18F‑C2Am, 2 h post‑administration. Mice bearing Colo205 tumors were treated with MEDI3039 (closed bars; 0.4 mg/kg, i.v., 24 h) or vehicle (open bars). 18F‑C2Am was injected (1 MBq, i.v.) and tissues collected post‑mortem 2 h following administration. Fraction of injected activity per gram of tissue (IA/g, %) (mean±SEM, n=5 per treatment group).
-
There was increased cell death in the spleen and liver following MEDI3039 treatment.
-
The blood half-life of 18F-C2Am, calculated from the analysis of dynamic data obtained from ROIs placed in the carotid artery, was 12.4±2.2 min. 18F-C2Am was stable in plasma for up to 8 h at 37 °C and was found to be intact in mouse blood in vivo at 15 min post-administration.
-
Dynamic PET imaging confirmed renal excretion as the predominant clearance route for 18F-C2Am. Bladder signal could be detected within 5–10 min of administration (Fig. 4a, b) and kidney cortical uptake peaked (at ~ 40% IA/g) within 20–30 min of injection (Fig. 4c, d, green line), but was minimal at 2 h postadministration (~ 3% IA/g;).
-
Tumor signal (Fig. 4a,b) could be detected within 15 min of injection, and there was a small component (< 10%) of hepatobiliary clearance (Fig. 4c, d, blue line). Tumor-to-muscle (T/m) and tumor-to-blood (T/b) ratios were 10.7±2.4 and 3.6.±0.5, respectively, for MDAMB-231 tumors and 6.1±2.1 and 2.4±0.4, respectively, for Colo205 tumors, at 2 h post-administration of 18F-C2Am. Analysis of individual tumors before and after treatment (Fig. 4e) showed significant increases in tumor retention (IA/g, %) of 18F-C2Am at 1 h and 2 h post-administration, for both models. This significant difference between pre- and posttreatment 18F-C2Am tumor uptake was also observed using SUV as the contrast metric (Fig. 4f). The tumor cells had been transduced with a viral vector expressing firefly luciferase prior to implantation, and both tumor models showed a significant decrease in bioluminescence post-treatment, where this decrease correlated with increased tumor uptake of 18F-C2Am in MDA-MB-231 (IA/g, %) and in Colo205 (T/b) tumors (Fig. 4g).
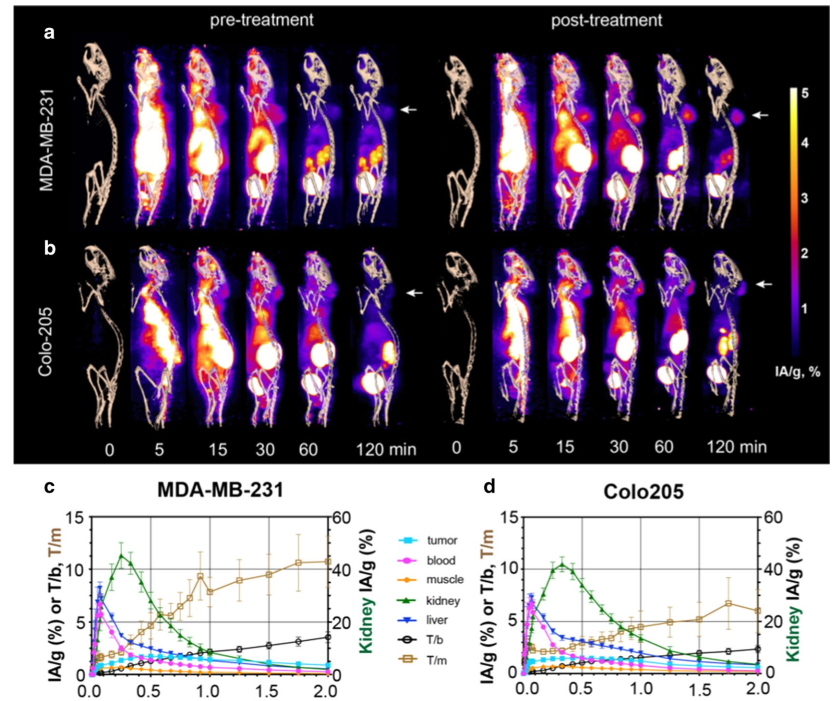
Fig. 4 PET images of 18F‑C2Am in tumor‑bearing mice, pre‑ and post‑treatment. Maximum intensity projections of the PET signal in sagittal
sections of representative mice, bearing MDA‑MB‑231 (a) and Colo205 (b) tumors. Projections are shown from immediately before (0) and up to 120 min after injection of 18F‑C2Am. Signal is shown as injected activity per gram of tissue (IA/g, %) and overlaid on a CT‑derived skeleton mask. White arrows indicate tumor location. Time–activity (IA/g, %) curves for the indicated tissues in treated (MEDI3039, 0.4 mg/kg, 24 h) MDA‑MB‑231 (c) and Colo205 (d) tumor‑bearing mice.
In conclusion, 18F-C2Am was demonstrated as a PET agent for imaging cell death in vivo that showed fast renal clearance, good tumor contrast post-treatment and acceptable dosimetry. 18F-C2Am has the potential to be used in the clinic to assess early treatment response in tumors, such as breast, prostate, lung and colorectal.
Full article on ejnmmires.springeropen.com
How can we help you?
Don't hesitate to contact us for technical information or to find out more about our products and services.
Get in touch

