Auger electron-emitting EGFR-targeted and non-targeted [197Hg]Hg-gold nanoparticles for treatment of glioblastoma multiforme (GBM)
2025.07.17.
Madeline K Brown et al, EJNMMI Radiopharm Chem. 2025
Summary
Background: Radiation nanomedicines are described here for glioblastoma multiforme (GBM) composed of gold nanoparticles (AuNPs) that integrate the Auger electron-emitter, 197Hg. [197Hg]Hg-AuNPs were conjugated to anti-epidermal growth factor receptor (EGFR) panitumumab or were non-targeted. The aim was to compare the cytotoxicity and DNA-damaging properties in vitro of panitumumab-[197Hg]Hg-AuNPs and non-targeted [197Hg]Hg-AuNPs on U251-Luc human GBM cells and estimate their cellular dosimetry. Additional aimwas to compare the biodistribution in vivo of panitumumab-[197Hg]Hg-AuNPs and [197Hg]Hg-AuNPs after convection-enhanced delivery (CED) in NRG mice with U251-Luc tumours in the brain and estimate the absorbed doses in the tumour and surrounding margins of healthy brain.
Results: [197Hg]Hg-AuNPs (26.8 ± 6.4 nm) were produced with a radiochemical yield of 98 ± 1% by incorporating 197Hg into the Turkevich synthesis method, forming a mercury-gold amalgam. Panitumumab-[197Hg]Hg-AuNPs exhibited high affinity binding to EGFR-positive U251-Luc cells. The binding of panitumumab-[197Hg]Hg-AuNPs to U251-Luc cells was 15-fold higher than [197Hg]Hg-AuNPs, and internalization and nuclear uptake were 12-fold and 18-fold greater, respectively. Panitumumab-[197Hg]Hg-AuNPs caused 84-fold more DNA double-strand breaks (DSBs) in U251-Luc cells than [197Hg]Hg-AuNPs. Panitumumab-[197Hg]Hg-AuNPs were ninefold more effective at reducing the clonogenic survival of U251-Luc cells than [197Hg]Hg-AuNPs. Panitumumab-[197Hg]Hg-AuNPs were twofold more cytotoxic than non-radioactive panitumumab-AuNPs and fivefold more cytotoxic than panitumumab. The absorbed doses in the nucleus of U251-Luc cells treated in vitro with panitumumab-[197Hg]Hg-AuNPs or [197Hg]Hg-AuNPs were 8.8 ± 2.9 Gy and 0.6 ± 0.1 Gy, respectively. SPECT/CT imaging showed that panitumumab-[197Hg]Hg-AuNPs and [197Hg]Hg-AuNPs were strongly retained at the infusion site in the brain after CED up to 7 d in NRG mice with orthotopic U251-Luc tumours. Uptake of panitumumab-[197Hg]Hg-AuNPs in the tumour-bearing right hemisphere was 172-fold and 579-fold greater than in the healthy left hemisphere and cerebellum, respectively. The uptake of [197Hg]Hg-AuNPs in the tumour-bearing right hemisphere was 85-fold and 64-fold higher than the left hemisphere and cerebellum, respectively. Most normal tissue uptake was < 1% ID/g, except for kidneys, spleen and liver. Dosimetry showed that 58% of the tumour received > 190 Gy for CED of 1.0 MBq of panitumumab-[197Hg]Hg-AuNPs vs. 0.6% of the tumour for non-targeted [197Hg]Hg-AuNPs, but both agents deposited > 50 Gy in 95% of the tumour. Doses decreased dramatically to 1.7 and 3.3 Gy at 1–3 mm from the tumour edge for panitumumab-[197Hg]Hg-AuNPs and [197Hg]Hg-AuNPs, respectively.
Conclusion: Radiation nanomedicines incorporating the AE-emitter, 197Hg administered by CED are a promising approach to treatment of GBM. Panitumumab-[197Hg]Hg-AuNPs are particularly attractive due to their EGFR-mediated binding, internalization and nuclear importation in GBM cells, which amplifies their in vitro cytotoxicity.
Results from nanoScan® SPECT/CT
- strong retention at the infusion site: SPECT/CT imaging demonstrated that both panitumumab-[¹⁹⁷Hg]Hg-AuNPs (EGFR-targeted) and non-targeted [¹⁹⁷Hg]Hg-AuNPs remained strongly localized at the convection-enhanced delivery (CED) site in the brains of NRG mice with orthotopic U251-Luc glioblastoma tumors up to 7 days post-infusion.
- Minimal redistribution elsewhere: The images showed very low activity outside the tumor infusion site — there was no detectable activity in other brain regions or major normal organs on SPECT/CT, indicating that the nanoparticles were largely confined locally after delivery.
- Greater diffusion of non-targeted particles: Closer examination of the SPECT/CT images revealed that non-targeted [¹⁹⁷Hg]Hg-AuNPs appeared to diffuse farther from the infusion site than the panitumumab-targeted nanoparticles, which remained more tightly localized, suggesting potential effects of target binding and cellular uptake on particle distribution.
- High uptake in tumor-bearing hemisphere: Quantitative biodistribution data derived in part from SPECT/CT corroborated that the tumor-bearing hemisphere had very high uptake of both targeted and non-targeted particles compared with the normal left hemisphere and cerebellum, with hundreds-fold higher % injected dose per gram in tumor tissue versus normal brain.
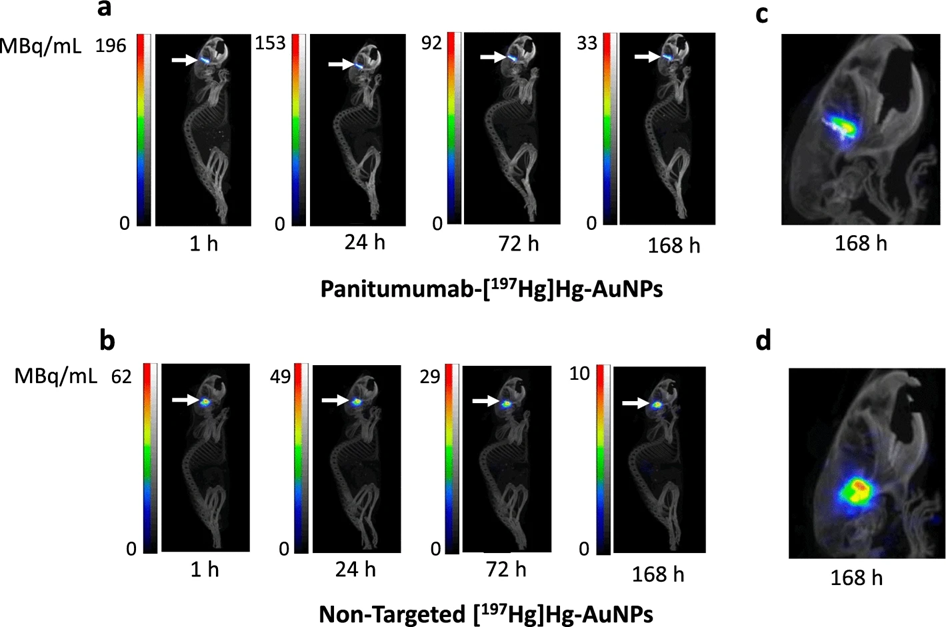
Figure 5. SPECT/CT images of NRG mice with orthotopic U251-Luc human GBM tumours in the right hemisphere of the brain administered a panitumumab-[197Hg]Hg-AuNPs or b non-targeted [197Hg]Hg-AuNPs by CED (arrows) at selected times up to 168 h post-infusion (p.i.). Intensity scales (MBq/mL) are shown for each timepoint post-infusion. Magnified view of the head showing differences in local diffusion from the infusion site in the brain for c panitumumab-[197Hg]Hg-AuNPs or d non-targeted [197Hg]Hg-AuNPs

Figure 6. Biodistribution of panitumumab-[197Hg]Hg-AuNPs or non-targeted [197Hg]Hg-AuNPs at 168 h p.i. The tumour could not be excised from the brain and was included in the right hemisphere tissue while the left hemisphere and cerebellum were non-tumour bearing. Values shown are the mean ± SD. There were no significant differences in the biodistribution of panitumumab-[197Hg]Hg-AuNPs and non-targeted [197Hg]Hg-AuNPs at this timepoint
Full article on link.springer.com
How can we help you?
Don't hesitate to contact us for technical information or to find out more about our products and services.
Get in touch
