Maleic anhydride derived diphosphines: adaptable chelators for receptor-targeted 99mTc, 64Cu and 188Re radiotracers
2025.08.26.
Rachel E. Nuttall et al., Chemical Science, 2025
Summary
In oncology, receptor-targeted molecular imaging using Single Photon Emission Computed Tomography (SPECT) or γ-scintigraphy has utility in diagnosis, disease staging and clinical decision-making. One class of radiotracer used for this purpose consists of a peptide attached to a chelator, which in turn is complexed to a radioactive metal ion. Two of the most prominent SPECT radiotracers employed for this purpose are 111In-DTPA-octreotide and 99mTc-EDDA/HYNIC-octreotide: both target the somatostatin receptor 2 that is overexpressed in neuroendocrine cancers. Whilst SPECT/γ-scintigraphy procedures with these radiotracers have been superseded in some healthcare settings by more sensitive Positron Emission Tomography (PET) imaging coupled with the PET radiotracer 68Ga-DOTA-octreotate, they are still widely used in many clinics where PET is not available.
There are several factors that have led to the prevalence of SPECT/γ-scintigraphy imaging procedures. First, worldwide, there is simply more SPECT and γ-scintigraphy infrastructure than PET infrastructure, including in lower and middle income countries. Second, 99mTc (t½ = 6 h; 90% γ, 140 keV) is widely distributed and available from bench-top 99Mo/99mTc generators in the form of 99mTcO4− in aqueous saline solution. Third, 99mTc radiotracers, which are routinely used in 30–40 million procedures globally each year, are produced using simple one- or two-step kits in near-quantitative radiochemical yields.
Kits for the preparation of 99mTc radiopharmaceuticals typically contain buffering salts, a reducing agent to reduce 99mTcVIIO4−, a chelator derivative that ultimately complexes the 99mTc metal ion to form the desired radiopharmaceutical, and other reagents including weak chelators, which serve to stabilise 99mTc intermediates. The majority of 99mTc radiopharmaceuticals are for imaging perfusion or anatomical processes. Molecular imaging using 99mTc-EDDA/HYNIC-octreotide is not as prevalent, in part because of the low incidence of neuroendocrine cancer.
However, recognising the utility and availability of molecular SPECT/γ-scintigraphy infrastructure, in recent years, several new 99mTc-labelled peptides have been clinically evaluated for receptor-targeted molecular imaging of the prostate-specific membrane antigen (PSMA) overexpressed in prostate cancer. These include 99mTc-MIP-1404 (also known as 99mTc-Trofolastat), 99mTc-PSMA-I&S and 99mTc-EDDA/HYNIC-iPSMA, which have been shown to be viable alternatives to efficacious PET diagnostic agents that similarly target PSMA.
There is an additional incentive for development of 99mTc radiotracers: in some cases, chemically analogous Re complexes are accessible, providing access to pairs of “theranostic” 99mTc and 188Re radiopharmaceuticals for diagnosis and therapy, respectively. The rhenium radionuclide, 188Re (t½ = 17 h; 100% β−, Emax = 2.12 MeV; 15% γ, 155 keV), which can also be produced from a bench-top generator like 99mTc, emits cytotoxic β− particles. 188Re radiopharmaceuticals can be effective therapeutics. For example, the lipophilic 188Re-labelled radiopharmaceutical 188Re-lipiodol is not only clinically efficacious for treatment of inoperable liver cancer, but is also economically viable in lower and middle income countries where supplies of other β−-emitting radiopharmaceuticals are limited due to economical and/or geographical barriers. Indeed, a newly developed pair of 99mTc/188Re-labelled PSMA-GCK01 theranostic agents has shown favourable properties in preclinical and initial first-in-human studies, demonstrating the feasibility of molecular imaging and therapeutic 99mTc/188Re pairs.
The authors have recently explored diphosphine derivatives as potential platforms for radiolabelling receptor-targeted biomolecules. These diphosphines include 2,3-bis(diphenylphosphino)maleic anhydride (DPPh) and 2,3-bis(di-p-tolylphosphino)maleic anhydride (DPTol) (Fig. 1) which react with the primary amine groups of peptides to furnish diphosphine-peptide (DP-peptide) conjugates. The DP-peptide derivatives coordinate [TcO2]+ or [ReO2]+ motifs to yield complexes of the type cis/trans-[MO2(DP-peptide)2]+ (M = Tc, Re); radiolabelled 99mTc and 188Re isotopologues are also synthetically accessible. They have also demonstrated that the resulting 99mTc and 188Re radiotracers retain affinity for their cognate target receptors in vitro and in vivo, and have favourable biodistribution pathways including rapid clearance from the bloodstream via a renal pathway. The authors note that these derivatives also coordinate the PET imaging isotope, 64Cu (t½ = 12.7 h; 18% β+, Emax = 653 keV), rapidly at room temperature, furnishing radiotracers of formula [Cu(DP-peptide)2]+ that show high stability in serum.
However, DP-peptide derivatives of DPPh do not provide 99mTc radiotracers in sufficiently high radiochemical yield to enable preparation using a one-step kit without subsequent purification to remove unreacted 99mTc precursors. Furthermore, radiochemical yields of 188Re derivatives are relatively low (<50%). DP-peptide derivatives of DPTol (which contains p-tolyl substituents in place of phenyl groups) have shown increased electron donor capacity and concomitant increased radiochemical yields compared to DPPh derivatives. However, even with the improved reactivity of DPTol, the authors have not been able to obtain radiochemical yields of [99mTcO2(DP-peptide)2]+ compounds above ∼85–90%. This is a barrier to clinical translation.
To address this, the authors have synthesised two novel diphosphine maleic anhydrides, DPAn and DPMEP (Fig. 1), which possess either p-anisyl or p-(2-methoxyethoxy)phenyl groups on the phosphines, respectively. Bioconjugates of these diphosphine maleic anhydride platforms enable near-quantitative radiochemical syntheses of 99mTc radiotracers. In their most comprehensive report yet, they have explored the scope of possible bioconjugation reactions with both DPAn and DPMEP using a range of biological targeting vectors. They have attached DPAn and DPMEP to a PSMA-targeted dipeptide (PSMAt), enabling comparison of DPAn-PSMAt and DPMEP-PSMAt with our prior conjugate, DPPh-PSMAt, including formation of 99mTc, 188Re and 64Cu complexes. Lastly, our novel 99mTc radiotracers have been assessed in murine models of prostate cancer using SPECT/CT imaging. The authors have therefore demonstrated the full utility of our novel and improved DPAn and DPMEP platforms for enabling economical and accessible production of pairs of receptor-targeted theranostic radiotracers.
Results from nanoScan® SPECT/CT
SPECT/CT scanning and biodistribution studies: The 99mTc radiotracers (3.9–4.5 MBq, 3 mice per group, containing DPAn-PSMAt = 3.0 µg or DPMEP-PSMAt = 3.5 µg) were administered via tail vein injection under isoflurane anaesthesia. Following this, SPECT/CT scanning was carried out in one of two methods outlined below, to maintain 2 h biodistribution timepoints:
- The mouse was positioned on a single heated bed in a nanoScan SPECT/CT 80W scanner (Mediso Ltd., Budapest, Hungary) calibrated for 99mTc. A helical CT scan was acquired (50 kV X-ray source, 170 ms exposure time in 360 projections over 3 min). At 15 min post-injection, whole body SPECT scans were acquired (1 × 15 min, 3 × 30 min, conducted sequentially) with a frame time of 13 s and 26 s (using a 4-head scanner with 4 × 9 [1.4 mm] pinhole collimators. in helical scanning mode). At 2 h post-injection, the animals were euthanized by cervical dislocation, organs/tissues harvested and weighed, and radioactivity counted using a gamma counter.
- The mouse remained under isoflurane anaesthesia on a heated bed, followed by euthanasia by pentobarbital injection at 2 h post-injection. The mouse was then scanned using a nanoScan SPECT/CT 80W scanner (Mediso Ltd., Budapest, Hungary) calibrated for 99mTc. A helical CT scan was acquired (50 kV X-ray source, 170 ms exposure time in 360 projections over 3 min). Subsequently, a 30 min whole-body SPECT scan was acquired with a frame time of 45 s (using a 4-head scanner with 4 × 9 [1.4 mm] pinhole collimators in helical scanning mode). After scanning, the organs/tissues were harvested, weighed and radioactivity counted using a gamma counter.
Quantification of SPECT/CT images (acquired at 15–30 min, 30–60 min, 60–90 min and 90–120 min post-injection, Fig. 8) indicated that tumour uptake of [99mTcO2(DPAn-PSMAt)2]+ was higher than that of [99mTcO2(DPMEP-PSMAt)2]+, consistent with in vitro uptake studies and ex vivo biodistribution data (vide infra). Additionally, for the mouse administered [99mTcO2(DPAn-PSMAt)2]+, the 99mTc tumour concentration noticeably increased from 30 min until 2 h post-injection.
SPECT/CT indicated that 99mTc residualised in salivary glands for animals administered either [99mTcO2(DPAn-PSMAt)2]+ or [99mTcO2(DPMEP-PSMAt)2]+. Two well-documented mechanisms of radiotracer uptake likely account for salivary gland uptake:
- PSMA is expressed at low levels in the salivary glands, kidneys and small intestine, with PSMA-targeted radiotracers showing uptake in the salivary glands, in both mice and human patients. Indeed, the salivary glands are considered dose-limiting organs, and uptake of radiotherapeutic PSMA-targeted radiopharmaceuticals can lead to xerostomia in prostate cancer patients.
-
TcO4−, bearing a single negative charge and with a similar polyatomic radius to that of the iodide anion, acts as a substrate for the sodium iodide symporter in vivo, and therefore accumulates in organs expressing the sodium iodide symporter – in mice, this includes the thyroid, salivary glands and stomach. SPECT imaging quantification (consistent with biodistribution data, vide infra) indicated that 99mTc concentrations were higher in the salivary glands for the mouse administered [99mTcO2(DPMEP-PSMAt)2]+ compared to the mouse administered [99mTcO2(DPAn-PSMAt)2]+. Indeed, 99mTc concentrations in the salivary glands increased over time for the former subject. This suggests that [99mTcO2(DPMEP-PSMAt)2]+ could possess lower in vivo stability than [99mTcO2(DPAn-PSMAt)2]+, with complex dissociation and oxidation of the [99mTcVO2]+ motif resulting in formation of 99mTcO4−in vivo, and increased 99mTc accumulation in the salivary glands for animals administered [99mTcO2(DPMEP-PSMAt)2]+.
The biodistribution of [99mTcO2(DPAn-PSMAt)2]+ and [99mTcO2(DPMEP-PSMAt)2]+ was further studied in (i) male SCID/Beige mice bearing DU145-PSMA+ prostate cancer xenografts (Fig. 8) and (ii) male nude mice bearing PSMA-expressing LNCaP prostate cancer xenografts (see SI).
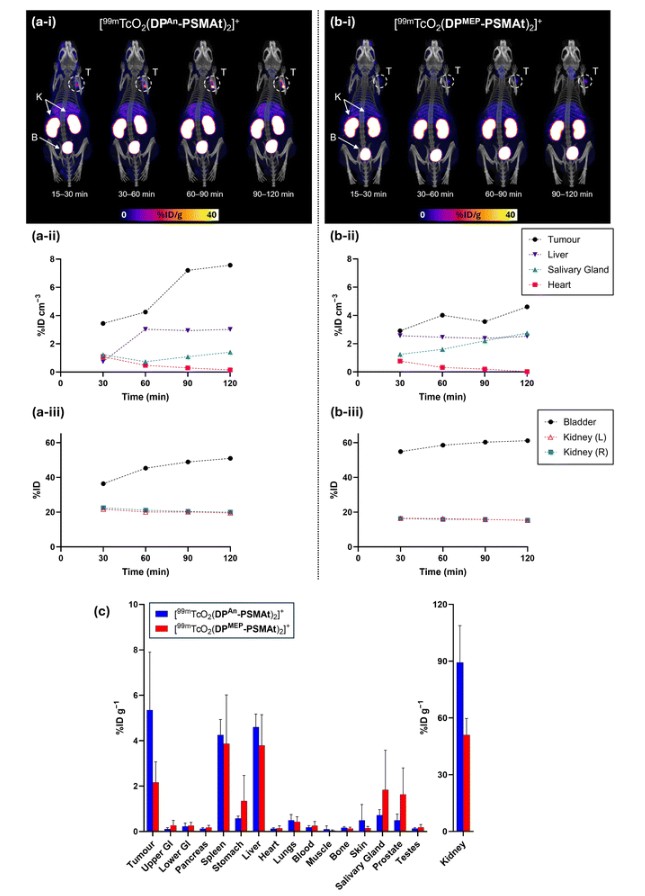
For all animals, significant concentrations of 99mTc radioactivity (2–5 %ID g−1) were measured in PSMA-expressing tumours 2 h post-injection, and although 99mTc tumour concentrations were consistently higher for animals administered [99mTcO2(DPAn-PSMAt)2]+, there were no statistically significant differences between [99mTcO2(DPAn-PSMAt)2]+ and [99mTcO2(DPMEP-PSMAt)2]+ in either mouse model. For both tracers, there were also significant concentrations of 99mTc radioactivity in the spleen, which is known to express low levels of PSMA and accumulate PSMA-targeted radiotracers, and the liver. Importantly, clearance from the blood pool and subsequent excretion was largely via a renal route, with concentrations of 40–90 %ID g−1 measured in kidneys.
The authors also noted that for both SCID/beige and nude mice studies, higher 99mTc concentrations were observed in the stomach and salivary glands for animals administered [99mTcO2(DPMEP-PSMAt)2]+ compared to animals administered [99mTcO2(DPAn-PSMAt)2]+, although these differences were not statistically significant. This observation is consistent with observations from SPECT/CT imaging studies, and further supports our conjecture that [99mTcO2(DPMEP-PSMAt)2]+ has lower in vivo stability than [99mTcO2(DPAn-PSMAt)2]+, with in vivo formation of 99mTcO4− from dissociation of [99mTcO2(DPMEP-PSMAt)2]+ resulting in higher levels of 99mTc activity in organs expressing the sodium iodide symporter.
Overall, the in vivo biodistribution demonstrates that both [99mTcO2(DPAn-PSMAt)2]+ and [99mTcO2(DPMEP-PSMAt)2]+ are useful SPECT or γ-scintigraphy imaging agents for PSMA expression in prostate cancer.
- In summary, the authors' new and highly versatile bis(phosphino) maleic anhydride platforms, DPAn and DPMEP, enable facile preparation of diphosphine bioconjugates that can be simply radiolabelled with 99mTc for SPECT imaging, 64Cu for PET imaging and 188Re for systemic radiotherapy, leading to the possibility of theranostic radiotracers.
- Importantly, these 99mTc radiotracers can be prepared in high radiochemical yields (≥95%) using a single step kit. This is a critical advance upon our prior DPPh and DPTol technology, as it allows for simple, one-step formulation of peptide-based 99mTc radiotracers, and presents new opportunities for economical molecular imaging using widely available 99mTc production infrastructure and γ-scintigraphy/SPECT cameras.
- The authors are currently expanding the application of DPAn and DPMEP chemistry to other therapeutically relevant receptor targets, to develop a versatile suite of molecular SPECT, PET and radiotherapeutic tracers.
How can we help you?
Don't hesitate to contact us for technical information or to find out more about our products and services.
Get in touch