Simultaneous Visualization of 161Tb- and 177Lu-Labeled Somatostatin Analogues Using Dual-Isotope SPECT Imaging
2021.04.12.
Francesca Borgna et al., MDPI Pharmaceutics, 2021
Summary
177Lu (half-life 6.7d) is currently the most often applied radiometal for therapeutic purposes, as it has a particulate emission (β− or Auger electron) for effecting therapy and emits several accompanying γ-photons of 208 keV (11%) and 113 keV (6.4%), which are used for diagnostic evaluation and dosimetry.
161Tb is a more recently introduced radiolanthanide for therapeutic applications. 161Tb decays with a half-life of 6.89 days to stable 161Dy, while emitting β¯-particles (Eβ͞av = 154 keV) suitable for therapeutic purposes and γ-radiation (Eγ = 49 keV, I = 17.0%; Eγ = 75 keV, I = 10.2%) useful for SPECT imaging. 161Tb also emits a substantial number of low-energy conversion and Auger electrons, which makes this radionuclide exceptionally interesting for the treatment of disseminated cancers with multiple metastases ranging from a single cell (diameter: ~10μm) to micro cell clusters (diameter: < 1mm). Monte Carlo simulations assessed the dose delivered to 10μm spheres revealed a 3.5-fold increased value when using 161Tb as compared to 177Lu. In larger tumors (diameter > 10mm), the emitted electron energy from 161Tb and 177Lu respectively is almost entirely absorbed, resulting in a 1.3-fold higher absorbed electron energy fraction per decay for 161Tb compared to 177Lu, making 161Tb more potent than 177Lu.
The aim of the present study was to use dual-isotope SPECT imaging in order to demonstrate that 161Tb and 177Lu are interchangeable without compromising the pharmacokinetic profile of the radiopharmaceutical.
After in vitro characterization, 161Tb- and 177Lu-labeled somatostatin (SST) analogues DOTATOC (agonist) and DOTA-LM3 (antagonist) were injected to AR42J tumor-bearing nude mice. In vivo disptribution profiles were investigatd by dual-isotope SPECT/CT imaging. Results revealed identical pharmacokinetic profiles of the two peptides, irrespective of whether it was labeled with 161Tb or 177Lu. Moreover, the visualization of the sub-organ distribution confirmed similar behavior of 161Tb- and 177Lu-labeled SST analogues. These and previous findings suggest that any future (pre)clinical studies with 161Tb can be based on preclinical data obtained with its 177Lu-labeled counterpart. This will allow the focusing of future investigations directly on the therapeutic efficacy of 161Tb, which is likely to be superior to the effect obtained with 177Lu.
Results from nanoScan® SPECT/CT
Five-week-old female CD1 nude mice were subcutaneously inoculated with AR42J tumor cells (5x106 cells in 100µl PBS). The scans were performed 10–14 days after tumor cell inoculation when the tumor size reached a volume of ~250mm3.
Mice were i.v. injected with a mixture of 161Tb-DOTATOC (~15MBq) and 177Lu-DOTATOC (~15MBq) or a mixture of 161Tb-DOTA-LM3 and 177Lu-DOTA-LM3 (~30MBq) at a 161Tb/177Lu activity ratio of 1:1. For specificity test, blocking studies were performed under the same experimental conditions; however, in this case, an excess of unlabeled DOTATOC or DOTA-LM3 was added to the injection solution. SPECT/CT scans were acquired 2h, 4h, and 24h after injection of the radiopeptides using the dual-isotope SPECT acquisition protocol with a frame time of 60s resulting in a scan time of 45min.
For the SPECT/CT scan, simultaneous acquisition of counts stemming from 161Tb and 177Lu, respectively, was performed by the selection of distinct energy windows for the two radionuclides. The two energy windows chosen for 161Tb were set at 47.7keV±10%, which enabled the detection of X-rays and γ-rays (46.0keV, 48.9keV and 52.0keV), and at 74.6keV±10%, enabling the detection of the γ-rays at 74.6keV. For 177Lu, the windows were set at 112.9keV±10% and 208.4±10% to detect the γ-rays. Prior to animal studies, phantom scans were carried out in order to verify of the dual-isotope SPECT imaging protocol using Eppendorf vials filled with 161Tb or 177Lu or both: analysis revealed no interference between 161Tb and 177Lu in the acquired scans; each radionuclide was visualized independently of the other with high accuracy.
Analysis of the SPECT/CT images revealed:
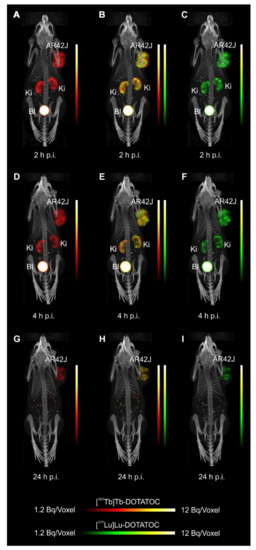
- Equal in vivo distribution of simultaneously injected 161Tb-DOTATOC and 177Lu-DOTATOC. The same observation was made for 161Tb-DOTA-LM3 and 177Lu-DOTA-LM3. Images reconstructed using the energies of either radiolanthanide (red-to-yellow scale and green-to-yellow scale for 161Tb and 177Lu, respectively), provided the distribution for each radiopeptide separately in the same mouse.
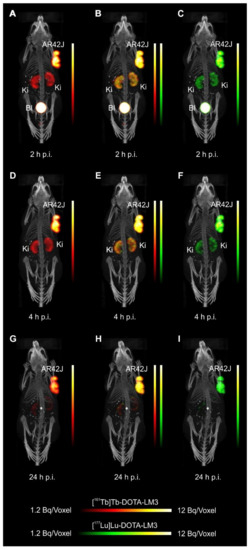
- Experiments performed by co-injection of excess unlabeled peptide resulted in SSTR blockade and, hence, accumulation of the radiopeptides in AR42J tumors was not observed. These additional studies proved that the uptake of the SST analogues in AR42J tumor xenografts was SSTR-specific.
- Quantification of the accumulated activity in AR42J tumors and kidneys confirmed equal distribution of the 161Tb- and 177Lu-labeled counterparts. This was the ultimate proof that the chosen radiolanthanide did not have an impact on the tissue distribution profile of the radiopeptides.
- The SPECT/CT images showed activity accumulation in the AR42J xenografts, which was higher for the antagonist than for the agonist. In agreement with quantitative data from biodistribution studies, the activity was efficiently cleared through the kidneys over time and almost entirely excreted after 24h. Due to the favorable uptake of radiolabeled DOTA-LM3 in the tumor tissue, the tumor-to-kidney ratio was higher as compared to the ratio obtained after injection of radiolabeled DOTATOC.
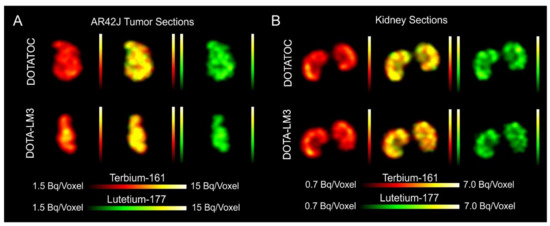
- Dual-isotope SPECT image sections enabled, for the first time, visualization of the 161Tb- and 177Lu-labeled peptide distribution at a sub-organ level in the same animal. Most important to note is that the pattern of activity distribution in tumors and kidneys was the same, irrespective of whether 161Tb or 177Lu was used. The uptake in the tumor was quite homogenous, which can be ascribed to the well-vascularized AR42J xenograft. Accumulation of activity in the kidneys was more prominent in the cortex where the megalin-mediated reabsorption of radiopeptides occurs, and where various SSTR subtypes are known to be expressed
Hogyan segíthetünk Önnek?
További termékinformációkért, vagy támogatásért keresse szakértőinket!
Vegye fel a kapcsolatot
