Design and Preclinical Evaluation of Novel uPAR-Targeting Radiopeptides Modified with an Albumin-Binding Entity
2025.05.06.
Darja Beyer et al., ACS Publications, 2025
Summary
The uPAR receptor was found to be overexpressed in many tumors and was identified as a promising target for diagnostic and therapeutic radiopharmaceuticals. The AE105 peptide was previously developed as a high-affinity uPAR ligand, but its therapeutic use was limited by rapid clearance and low tumor uptake. In this study, novel AE105-based radiopeptides with albumin-binding entities were developed to improve pharmacokinetics and tumor accumulation for radionuclide therapy and SPECT imaging. The 177Lu-labeled uPAR-targeting peptides were evaluated in preclinical in vitro and in vivo models.
Results from nanoScan® SPECT/CT
The mice were imaged using SPECT/CT at 1, 4, and 24 h after radiopeptide injection using a four-head nanoSPECT/CT® small-animal imaging system. CT scans were acquired for 7–9 min, followed by SPECT acquisitions lasting 45–50 min using a tungsten-based MPH collimator (9 × 1.4 mm pinholes) to achieve high spatial resolution. SPECT data were reconstructed using the characteristic γ-energies of 177Lu, and a Gaussian post-reconstruction filter was applied to reduce image noise. CT data were reconstructed using cone-beam FBP, and images were displayed with a quantitative activity scale.
The SPECT/CT images clearly demonstrated the prolonged blood retention of the albumin-binding radiopeptides, resulting in higher background activity compared to the rapidly cleared [177Lu]Lu-DOTA-AE105. High cardiac activity was observed at early time points, particularly for [177Lu]Lu-uPAR-03, which exhibited the longest circulation time, whereas [177Lu]Lu-uPAR-02 provided the most favorable tumor-to-background contrast over time. Tumor xenograft accumulation improved progressively with the albumin-binding radiopeptides, consistent with their enhanced metabolic stability (>98% intact after 4 h incubation in plasma), in contrast to the rapid degradation of [177Lu]Lu-DOTA-AE105. Differences among the novel radiopeptides could be attributed to their albumin-binding affinity and the presence of PEG or AMBA spacer entities, which modulated circulation time, metabolic stability, and tumor uptake, as reflected in the SPECT/CT images. (Fig.4.)
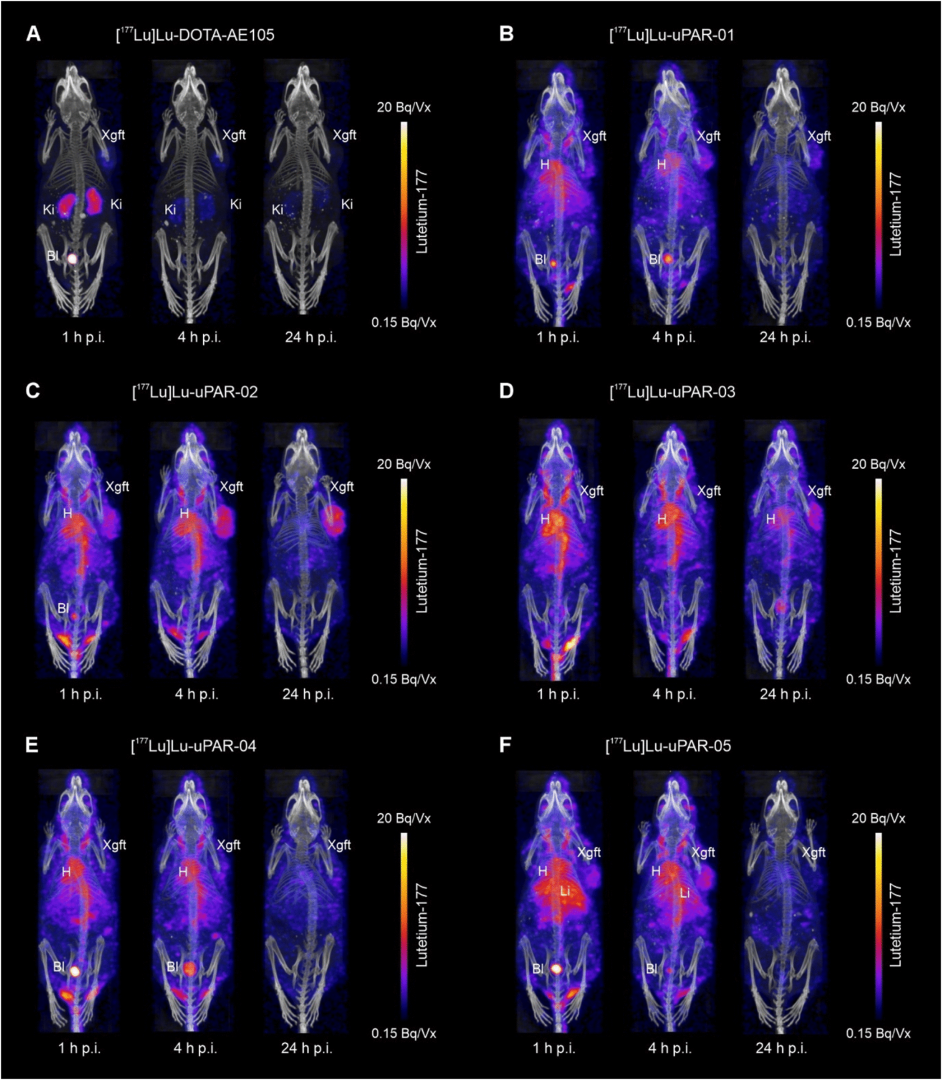
Figure 4. SPECT/CT of HEK-uPAR xenografts at 1, 4, and 24 h p.i. of [177Lu]Lu-DOTA-AE105 and [177Lu]Lu-uPAR-01–05. Bl, bladder; H, heart; Ki, kidneys; Xgft, xenograft; Vx, voxel.
Conclusion
SPECT/CT imaging demonstrated that albumin-binding uPAR-targeting radiopeptides achieve prolonged blood retention and higher tumor accumulation. Among the candidates, [177Lu]Lu-uPAR-02 showed the best combination of moderate albumin-binding, metabolic stability, and tumor-to-background contrast, making it the most promising for therapeutic and imaging applications.
Full article on pubs.acs.org
Hogyan segíthetünk Önnek?
További termékinformációkért, vagy támogatásért keresse szakértőinket!
Vegye fel a kapcsolatot