Design and Preclinical Evaluation of Novel uPAR-Targeting Radiopeptides Modified with an Albumin-Binding Entity
2025.05.06.
Darja Beyer et al., ACS Publications, 2025
Summary
The uPAR receptor was found to be overexpressed in many tumors and was identified as a promising target for diagnostic and therapeutic radiopharmaceuticals. The AE105 peptide was previously developed as a high-affinity uPAR ligand, but its therapeutic use was limited by rapid clearance and low tumor uptake. In this study, novel AE105-based radiopeptides with albumin-binding entities were developed to improve pharmacokinetics and tumor accumulation for radionuclide therapy and SPECT imaging. The 177Lu-labeled uPAR-targeting peptides were evaluated in preclinical in vitro and in vivo models.
Results from nanoScan® SPECT/CT
The mice were imaged using SPECT/CT at 1, 4, and 24 h after radiopeptide injection using a four-head nanoSPECT/CT® small-animal imaging system. CT scans were acquired for 7–9 min, followed by SPECT acquisitions lasting 45–50 min using a tungsten-based MPH collimator (9 × 1.4 mm pinholes) to achieve high spatial resolution. SPECT data were reconstructed using the characteristic γ-energies of 177Lu, and a Gaussian post-reconstruction filter was applied to reduce image noise. CT data were reconstructed using cone-beam FBP, and images were displayed with a quantitative activity scale.
The SPECT/CT images clearly demonstrated the prolonged blood retention of the albumin-binding radiopeptides, resulting in higher background activity compared to the rapidly cleared [177Lu]Lu-DOTA-AE105. High cardiac activity was observed at early time points, particularly for [177Lu]Lu-uPAR-03, which exhibited the longest circulation time, whereas [177Lu]Lu-uPAR-02 provided the most favorable tumor-to-background contrast over time. Tumor xenograft accumulation improved progressively with the albumin-binding radiopeptides, consistent with their enhanced metabolic stability (>98% intact after 4 h incubation in plasma), in contrast to the rapid degradation of [177Lu]Lu-DOTA-AE105. Differences among the novel radiopeptides could be attributed to their albumin-binding affinity and the presence of PEG or AMBA spacer entities, which modulated circulation time, metabolic stability, and tumor uptake, as reflected in the SPECT/CT images. (Fig.4.)
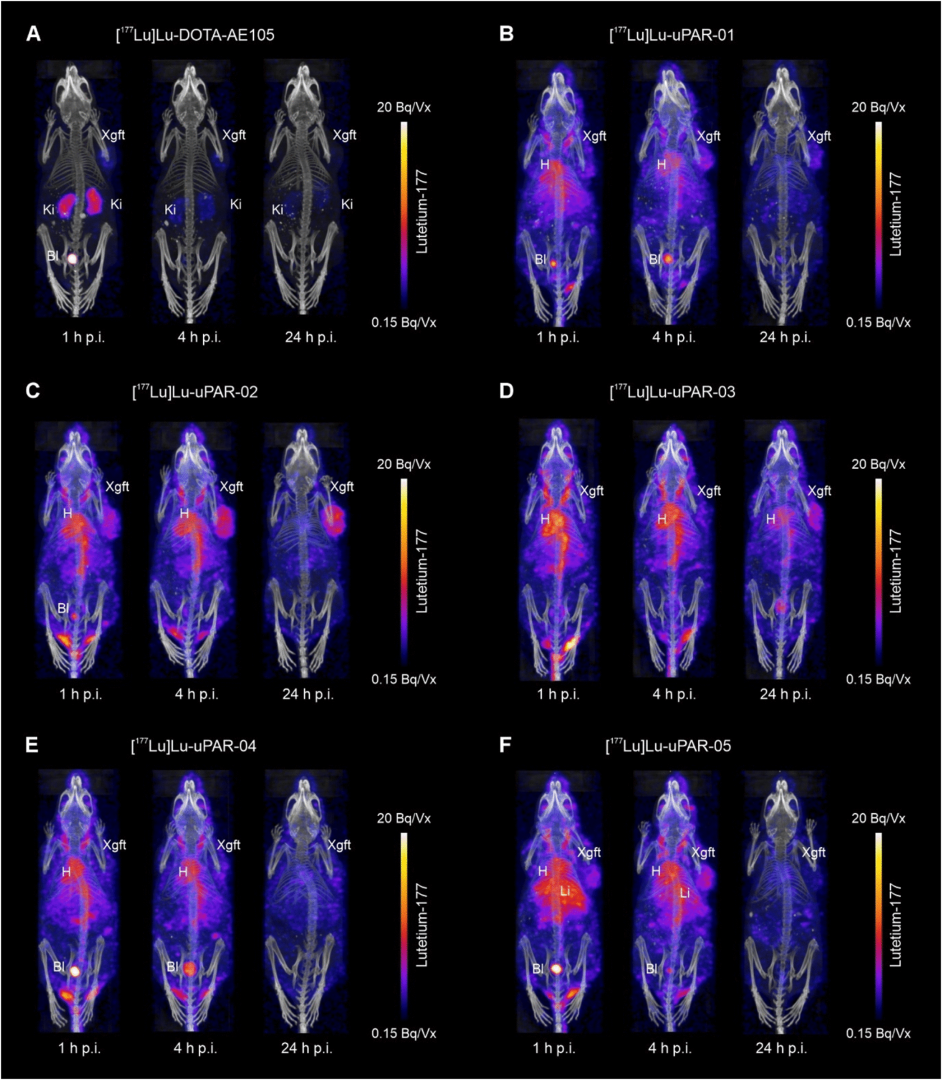
Figure 4. SPECT/CT of HEK-uPAR xenografts at 1, 4, and 24 h p.i. of [177Lu]Lu-DOTA-AE105 and [177Lu]Lu-uPAR-01–05. Bl, bladder; H, heart; Ki, kidneys; Xgft, xenograft; Vx, voxel.
Conclusion
SPECT/CT imaging demonstrated that albumin-binding uPAR-targeting radiopeptides achieve prolonged blood retention and higher tumor accumulation. Among the candidates, [177Lu]Lu-uPAR-02 showed the best combination of moderate albumin-binding, metabolic stability, and tumor-to-background contrast, making it the most promising for therapeutic and imaging applications.
Full article on pubs.acs.org
How can we help you?
Don't hesitate to contact us for technical information or to find out more about our products and services.
Get in touch