Rapid nanobody-based imaging of mesothelin expressing malignancies compatible with blocking therapeutic antibodies
2023.07.14.
Abdennour Benloucif et al.,Frontiers in Neuroscience, 2023
Summary
Advancements in cancer treatment emphasize precision medicine and combinatorial strategies, necessitating tools for accurate tumor evaluation and follow-up. Non-invasive molecular imaging, like optical imaging and PET, offers alternatives to biopsies. Nanobodies, with high affinity and rapid clearance, are promising probes for molecular imaging with a wide range of targets. Mesothelin (MSLN) is highly expressed in aggressive cancers, making it an attractive therapeutic target. However, reliable non-invasive imaging of MSLN-positive tumors is essential for patient stratification and monitoring treatment response. In this study, a high-affinity nanobody (S1) capable of specifically targeting MSLN-positive tumors was developed for optical and PET imaging. The nanobody demonstrated potential as a companion test for MSLN-targeting therapies, providing a step forward in cancer diagnosis and treatment monitoring.
The immune PET/CT imaging using [68Ga] Ga-NODAGA-S1 demonstrated successful targeting of MSLN-positive tumors in vivo, with rapid uptake and retention of the nanobody in the tumors. The time activity curves showed a significant uptake of the radiotracer in MSLN high tumors, while the signal decreased over time in MSLN low tumors. Ex vivo biodistribution analysis confirmed the specific uptake of [68Ga] Ga-NODAGA-S1 in MSLN high tumors compared to MSLN low tumors.
Results from nanoScan® PET/CT
A1847 MSLN pos and MDA-MB-231 MSLN low cells in a 1/1 (v/v) Matrigel suspension were implanted subcutaneously in 6-week-old female NMRI-Foxn1<nu>mice and grown until the tumours reached an average volume. The image acquisition was performed with NanoScan PET/CT camera (Mediso, Budapest, Hungary).
For dynamic micro PET/CT imaging, mice were under anaesthesia, and they injected on their tail vain with 68Ga-nanobody S1 (5-7 MBq/mouse). The acquisition time was 2 hours.
The static micro PET /CT imaging acquisition time was 20 minutes, after 2 hours uptake time. The injected radiotracer was 5 Mbq/ mouse.
The results highlight the potential of the anti-MSLN nanobody as a promising PET/CT imaging agent for non-invasive detection of MSLN-positive tumors. The high contrast images obtained shortly after systemic injection demonstrate the specificity and selectivity of the nanobody for MSLN-targeted imaging.
- The nanobody exhibited rapid targeting of MSLN+ tumors, with detectable signals just 10 minutes after injection, becoming clearly visible at 60 minutes, and remaining retained throughout the 120-minute scan period.
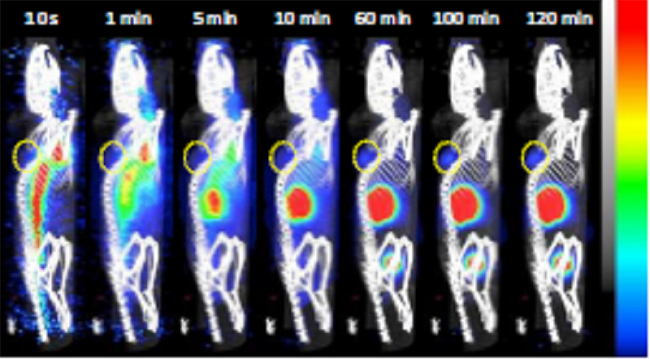
Figure 4. (A) Representative sagittal PET images of A1847 tumour-bearing mice after injection of [68Ga]Ga-NODAGA-S1 (5-7 MBq) during 2h dynamic scan. Yellow circle indicates the tumour
- The time activity curves for the tumors presented in revealed a fast uptake in A1847 tumors, which was sustained up to 2 hours after injection. In contrast, the uptake in MDA-MB-231 tumors decreased over time. Additionally, there was noticeable radioactivity accumulation in the kidneys and bladder, consistent with the well-known kidney retention and rapid blood clearance characteristics of the nanobody.

Figure 4. (B) Time activity curves (decay corrected) generated following radioactivity quantification from PET images (n=5 mice, A1847, n=3 mice, MDA-MB-231).
- After 2 hours post-injection, PET static scans were conducted on mice with A1847 or MDA-MB-231 tumors. The ex vivo biodistribution analysis clearly showed a remarkable increase in [68Ga]Ga-NODAGA-S1 uptake in MSLNhigh tumors (as depicted in Figure 4C) compared to the MSLNlow tumors with low expression or negative for MSLN. This finding highlights the specificity of [68Ga]Ga-NODAGA-S1 in targeting MSLN-positive tumors, offering promising potential for accurate tumor detection and stratification in cancer imaging applications.

Figure 4 (C) Ex vivo quantification of radioactivity in tumours. Data are expressed as a percentage of injected dose per gram of tissue after gamma-counting (n=14 mice for A1847 and 12 mice for MDA MB 231). Data were analysed using a two-tailed unpaired t-test: ***, p<0.001.
- The competition experiment with excess unlabeled nanobody (Nb) validated the specific uptake of [68Ga]Ga-NODAGA-S1 in MSLN-positive tumors. MSLNlow tumors showed no significant change in uptake, while MSLNhigh tumors exhibited a 50% decrease in signal, confirming the nanobody's selectivity for MSLN-positive tumors.

Figure 4 (D) Competition experiment: tumour-bearing mice (n=6/group) were injected with [68Ga]Ga-NODAGA-S1 (5-7 MBq) and microPET images were acquired during 20 min, 2 h after intravenous injection of the radiotracer. The next day, the same mice were pretreated with a 150-fold molar excess of unlabeled S1 (1 mg, i.v.) 20 min before injection of [68Ga]Ga-NODAGA-S1 (5-7 MBq) (n = 6 mice/group) and images were acquired during 20 min, 2 h after intravenous injection of the radiotracer. Analysis of the PET signal was performed on attenuation- and decay-corrected PET images. Data were analysed using a two-way ANOVA followed by Turkey post-test: ****, p<0.0001.
- The 68Ga-labeled nanobody showed high uptake in tumors, kidneys, and unexpectedly, in the liver and lung of 2-3 mice, possibly indicating emerging tumor foci.

Figure 4 (E) Ex vivo biodistribution profile of [68Ga]Ga-NODAGA-S1 at 2 h post-injection in A1847 and MDA-MB 231-xenografted mice (n=14 mice for A1847 and 12 mice for MDA MB 231
Moreover, the use of two different mouse strains for imaging experiments validated the nanobody's efficacy in murine models, regardless of their degree of immunodeficiency, suggesting its potential applicability in various preclinical models.
These findings pave the way for further investigations and the development of a theranostic approach using the anti-MSLN nanobody, where the diagnostic radioisotope could be substituted with a therapeutic isotope like 177Lu, providing a combined diagnostic and therapeutic strategy for MSLN-positive tumors. This approach holds promise for advancing cancer treatment and precision medicine, potentially benefiting patients with aggressive cancers expressing high levels of MSLN.
Full article on frontiersin.org
Hogyan segíthetünk Önnek?
További termékinformációkért, vagy támogatásért keresse szakértőinket!
Vegye fel a kapcsolatot
